Abstract
Background: Cardiotoxicity is one of the side effects of many antineoplastic treatments. Establishing a baseline cardiac function prior to initiating therapy is of paramount importance and may be the limiting factor to choose a certain antineoplastic treatment regimen. Echocardiographic strain techniques including Global Longitudinal Strain (GLS) measurements are sensitive for detecting preclinical cardiac dysfunction and is a predictor for future reduction in ejection fraction and long-term outcome. Autologous transplant is performed as a consolidation strategy for many hematological malignancies and patients who undergo transplant are treated with high doses of chemotherapy including alkylating agents, which can affect cardiac function along with the strain of pancytopenia. Literature that describes the effects of transplant on cardiac function in patients who have reduced Global Longitudinal Strain is scarce if any at all. Most of the data for low GLS in patients receiving cardiotoxic medications suggest poor long-term morbidity and mortality.
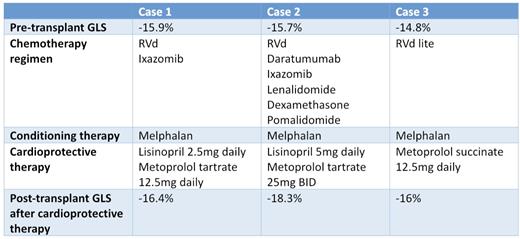
Objective and design: We present a case series of 3 patients diagnosed with multiple myeloma undergoing an autologous stem cell transplant as part of their treatment with reduced global longitudinal strain on pre-transplant assessment. These patients were treated with cardioprotective medications (Beta Blockers and/or ACEi/ARBs) before and while undergoing conditioning chemotherapy and subsequently autologous stem cell transplant and were followed until day 100.
Results: Follow up in the post-transplant period showed improvement of GLS with and none of these patients demonstrated any signs or symptoms of cardiovascular morbidity.
Conclusion: Reduced GLS should therapy in patients undergoing ASCT and cardioprotective medications may have a role in reducing cardiac morbidity and mortality in these patients. Our patients displayed improved GLS in the post-transplant period suggesting the need for further research and streamlining the role of cardio-oncology and cardioprotective treatment in similar situations.
Raval: Abbvie Pharmaceuticals: Speakers Bureau; Adaptive Biotechnologies: Consultancy; ADCT Therapeutics: Consultancy, Speakers Bureau; Alexion Pharmaceuticals: Speakers Bureau; Amgen Biotechnology Company: Research Funding; Astellas Pharmaceuticals: Speakers Bureau; Astrazeneca Pharmaceuticals: Consultancy, Speakers Bureau; Beigene Pharmaceuticals: Speakers Bureau; Bristol Meyers Squibb Pharmaceuticals: Consultancy; Epizyme Pharmaceuticals: Consultancy, Speakers Bureau; Genetech Biotechnology Company: Research Funding; GlaxoSmithKline Pharmaceuticals: Consultancy; Incyte Pharmaceuticals Corporation: Speakers Bureau; Jazz Pharmaceuticals: Consultancy; Karyopharm Therapeutics: Consultancy; Morphosys Biotech Company: Speakers Bureau; Sanofi Genzyme: Consultancy; Seagen Biotechnology Company: Research Funding; Takeda Pharmaceuticals: Consultancy, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal